Microsomal Stability
Assay information
Compound Requirements
10μL 10mM DMSO stock
Time Points
Screening: 0 and 15, 30, or 60 min
Half Life: 0, 5, 15, 30, and 60 min.
Species
Mouse
Rat
Human
*others available
Cofactor
NADPH
Quantitation Method
HPLC-MS
Data Delivery
Screening: Percent Remaining
Half Life: Intrinsic Clearance
Microsomes contain drug metabolizing enzymes and can indicate activity in future pharmacokinetic studies
Stability in microsomes is a suitable first step in metabolic stability screening technique due to it’s low cost and high-throughput. We’ve coupled high resolution accurate mass Q-TOF MS with HPLC to help you do more with less. Full-scan data is acquired, from which narrow window extracted ion chromatograms are generated, producing quantitative data equivalent to that obtained by HPLC/MS/MS without the time-consuming process of developing distinct, MS methods for each test article. Use this data to guide structural modifications, predict in-vivo performance, develop structure-metabolic stability relationships, and triage compounds for further studies in real time.
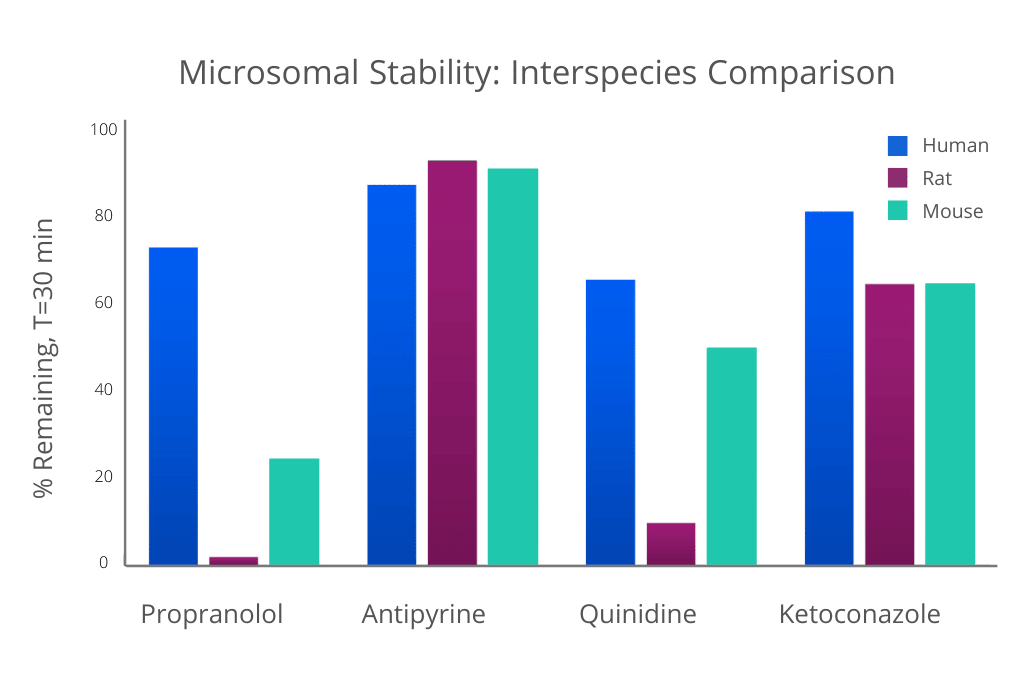
Results can vary between species; use in-vitro data to determine which species to use in pre-clinical studies. The data below was obtained at Analiza. Commercially available compounds were tested under the same conditions using human, rat, and mouse liver microsomes.
Contact us to learn more about our capabilities or to get a quote for your project.
